The brain is the most energy consuming organ of the body, yet it frequently experiences energetic stress during sleep, intense circuit activity, and fasting periods. We use a combination of unbiased and imaging-based approaches to discover novel mechanisms of metabolic adaptations in the nervous system, both in healthy and disease conditions.
Our research is focused on examining:
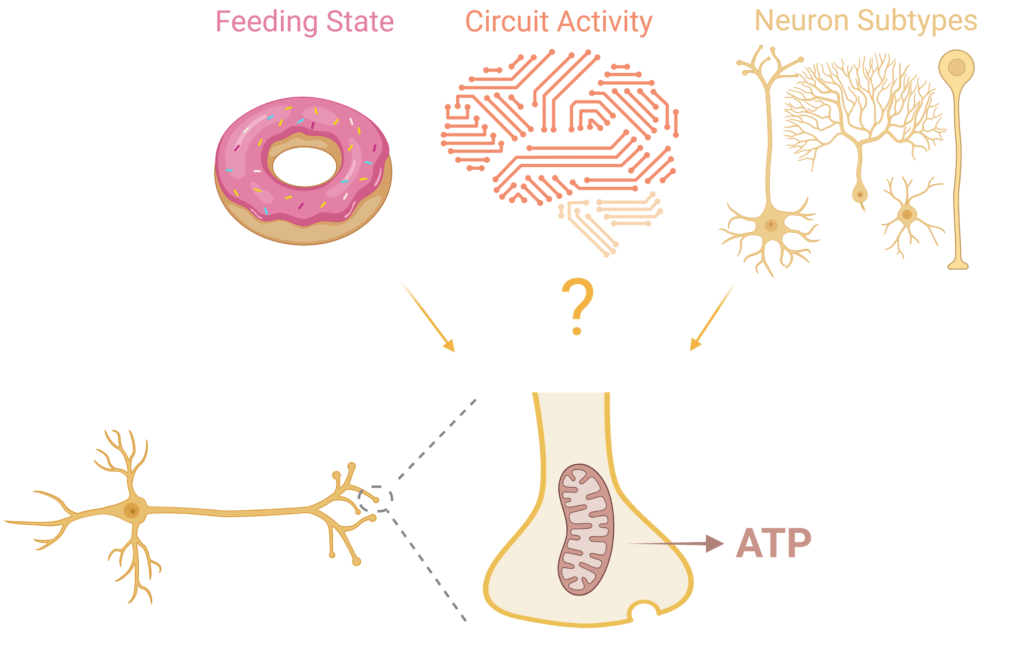
We employ a variety of state-of-the-art techniques, including, genomics, proteomics, and quantitative optical imaging to determine how energy metabolism is regulated in synaptic terminals. Recent studies from the lab have identified the role of Sirtuin 3 (Tiwari et al., 2024 JCB) and the mitochondrial pyruvate carrier (Tiwari et al., 2024 Sci Adv) in regulation of mitochondrial oxidative phosphorylation in synapses experiencing glucose deprivation. We have also shown that synaptic vesicle release and reuptake are differentially vulnerable to inhibition of mitochondrial and glycolytic ATP synthesis (Myeong et al., 2024 Cell Rep), highlighting the critical role of energy metabolism in the regulation of synaptic plasticity.
Dysregulation of neuronal bioenergetics is emerging as a critical factor in many neurodegenerative diseases. Our lab is a member of the Needleman Center for Neurometabolism and Axonal Therapeutics, where we leverage our expertise in functional imaging of single synapses to determine how metabolic dysregulation may lead to neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease. To this end, combine high throughput metabolomics with quantitative optical imaging in human iPSC-derived neurons. Our goal is to identify metabolic pathways whose dysregulation contribute to disease pathogenesis.
