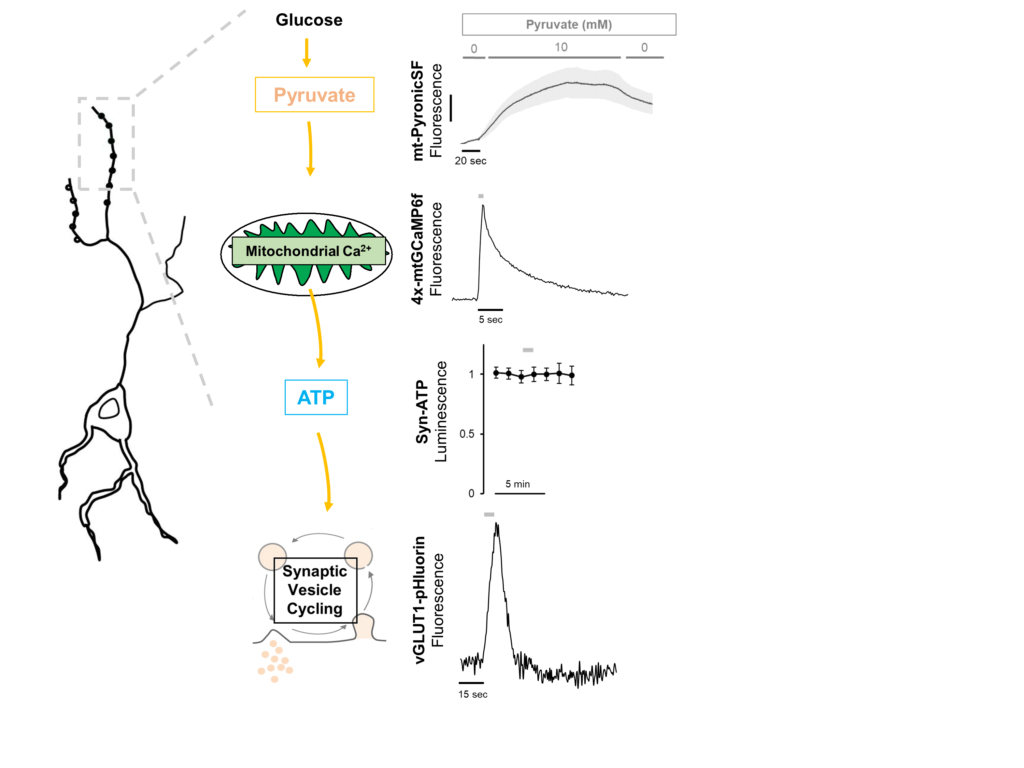
The brain is the most energy consuming organ of the body and is highly sensitive to energetic perturbations. But regulatory mechanisms that modulate neuronal metabolism are poorly understood. In firing nerve terminals, both glycolysis (Ashrafi et al., Neuron 2017) and oxidative phosphorylation (OXPHOS) (Ashrafi and de Juan-Sanz et al., Neuron 2020) are rapidly upregulated to meet the energetic requirements of synaptic activity. While this upregulation represents transient modulation of neuronal metabolism, it is not clear how neurons adapt to chronic changes in energetic burden that occur during intense electrical activity, fasting, or in response to disease states (such as neurodegeneration). We investigate how neurons utilize alternative fuels, such a ketones and lactate, when glucose concentration becomes limiting.
Our research is focused on uncovering:
In previous work, we demonstrated that glycolysis is upregulated in firing nerve terminals through recruitment of the glucose transporter GLUT4 to presynaptic membrane . Similarly, mitochondrial Ca2+ uptake during action potential firing acutely stimulates OXPHOS to increase ATP production . But how does persistent neuronal activity or changes in glucose availability alter the glycolytic and mitochondrial capacity of neurons for ATP production? We employ a variety of state-of-the-art techniques, including, genomics, proteomics, and quantitative optical imaging to determine how energy metabolism is regulated in synaptic terminals.
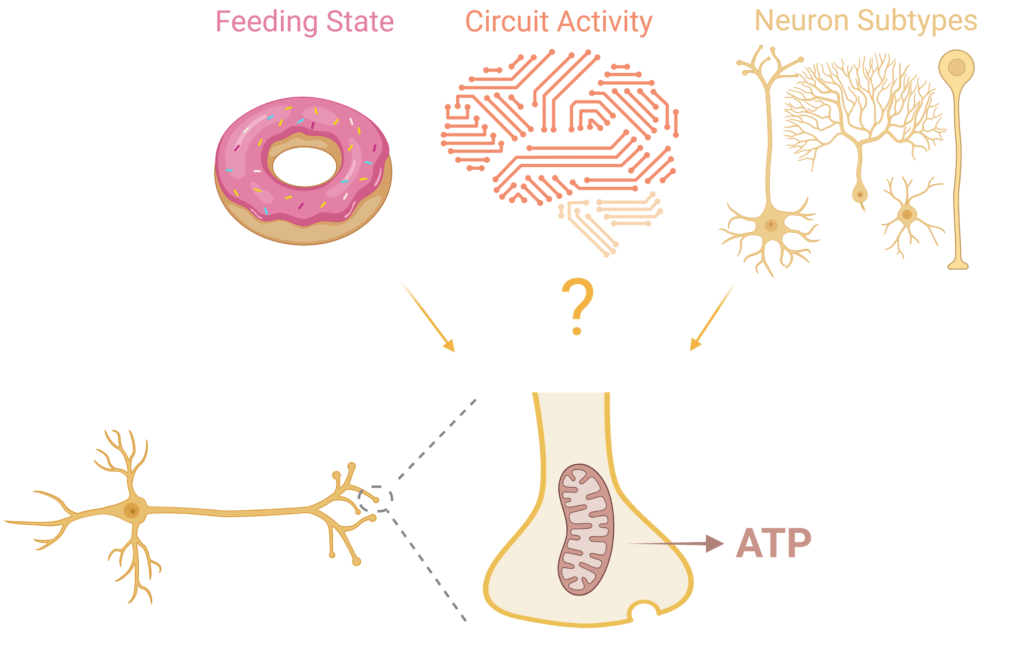
Dysregulation of neuronal bioenergetics is emerging as a critical factor in many neurodegenerative diseases. Therefore, it is important to determine the metabolic pathways that are impacted. As part of the Needleman Neurometabolism Center, we will extend our studies on acute and chronic regulation of neuronal metabolism to determine how metabolic dysregulation may lead to neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease. To this end, we will leverage our expertise in the application of optical sensors for quantitative measurement of metabolites in synaptic terminals. With this approach, we will identify the specific molecular pathways that are affected in different forms of neurodegeneration, and determine how such dysregulation may lead to neuronal loss.